Skin sensitisation tests are one of the current hot topics in the world of cosmetic ingredients evaluation. We are at the crossroad between animal and in vitro methods. What will be decided in the following months will definitively change the habits and evaluation approaches of manufacturers and toxicologists.
As an active actor that proposes solution for cosmetics industries, the IDEA TESTS Group is naturally part of this mutation.
A restrictive regulatory context
The main objective of the European Regulation (EC) 1223/20091 is the safety of a cosmetic product for human health as it is clearly announced in the article 3: A cosmetic product made available on the market shall be safe for human health when used under normal or reasonably foreseable conditions of use. In addition to the information collected on the finished product itself (type of product, target population, claims, application site, frequency, tests available, etc.), the toxicologist responsible for the safety evaluation has to look carefully at the toxicological profile of the substances, particularly for the calculation of the Margin of Safety (MOS).
The major parameters to be evaluated are described in the SCCS Note of Guidance for the testing of cosmetic substances and their safety evaluation2, in the REACH and CLP Regulation3,4, and are also mentioned in paragraph 8, Part A of the European Regulation (EC) 1223/2009: A particular focus on local toxicity evaluation (skin and eye irritation), skin sensitisation, and in the case of UV absorption photo-induced toxicity shall be made.
Therefore, the determination of skin sensitisation potential is one of the most important toxicological endpoint in the development and evaluation of ingredients used in fragrance, cosmetic and personal care products.
The basics of sensitisation
A skin sensitiser is a substance that will lead to an allergic response following skin contact.
Contact sensitisation is depending on the stimulation of an immune response and includes two phases: the first one, the induction, occurs when individual exposed to an allergen, develops cutaneous immune reaction and acquires a specialised immunological memory that could lead to a more aggressive secondary immune response if contact is made with the same allergen again. This secondary response is called elicitation and represents the second phase of the sensitisation, that is to say the production of a cell-mediated or antibody-mediated allergic response that leads to localised cutaneous inflammatory reaction at the site of skin exposure.
Tests carried on until March 2013
Due to the complexity of mechanistic reactions involved, the approaches used for the accurate identification and characterisation of chemicals that have the potential to cause skin sensitisation were, until March 2013, only based on animal testing. The most widely used were the Guinea Pig Maximisation Test (Magnusson & Kligman test - SMK), the Occluded Patch Test (Buehler test), both described in the OECD n° 406 Guideline5, and more recently the murine Local Lymph Node Assay (LLNA) described in the OECD No 429 Guideline6. The LLNA, performed on mice, was developed and used as an alternative to the GPMT and the Buehler tests.
One of the major advantages of the LLNA is that it provides a quantitative endpoint, dose-responsive data, and allows determining relative sensitising potency as a function of the vigor of induced proliferative responses.
March 2013: The animal testing ban
However, since March 11, 2013, animal use is totally banned in the European Union for cosmetics evaluation, even for repeated dose tests such as LLNA.
EU invested significantly since 2005 in the development of alternative in vitro method, particularly through the EU-FP6 project Sens-it-iv.
EURL ECVAM recently published a strategy for replacement of animal testing for skin sensitisation hazard identification and classification.8
The approach used was based on the AOP concept (Adverse Outcome Pathway).9 AOP concept relies with the analysis of every step of the mechanistic reactions involved in the sensitisation phenomenon, from the chemical structure and properties of the potential sensitiser, to the organism response.
AOP is summarised in the following scheme:
On this scheme LLNA can easily be placed at the organs response level and the SMK and Buhler tests at the organism response level, whereas, in vitro tests will be, by definition, only placed up to the cellular response level.
In addition, no specific step or reaction has been identified as a key event for the determination of the potency of an allergen.
This will obviously lead to the need of an accurate Integrated Testing Strategy for the full replacement of animal testing for skin sensitisation hazard identification and classification.
Thanks to the AOP approach and despite the mechanistic complexity of the endpoint, important advances in the development of alternative methods have been made. This progress is reflected in the numerous submissions of alternative test methods for skin sensitisation that EURL ECVAM has received.
Emerging alternative methods
At the first level of the skin sensitisation AOP, the in silico structure activity relationship approaches (SAR and/or QSAR), such as QSAR Toolbox 3.2 from OECD/ECHA, are being used since few years for the estimating of allergen properties of a substance on the basis of its molecular structure and the reactivity of its functional groups. These approaches are however quite complicated. They request well structured and extensive database and are only relevant for pure molecules.
Among the in vitro alternative methods described we can cite the followings: DPRA10, KeratinoSens11, LuSens (BASF), MUSST12, h-CLAT13, VitoSens14 (Vito-Cardam), Sens-IS15 (ImmunoSearch), Sensceetox16 (Ceetox), IL18 assessment17 and Skimune (Alcyomics). The endpoints and the analytical methods are summarised in the following table.
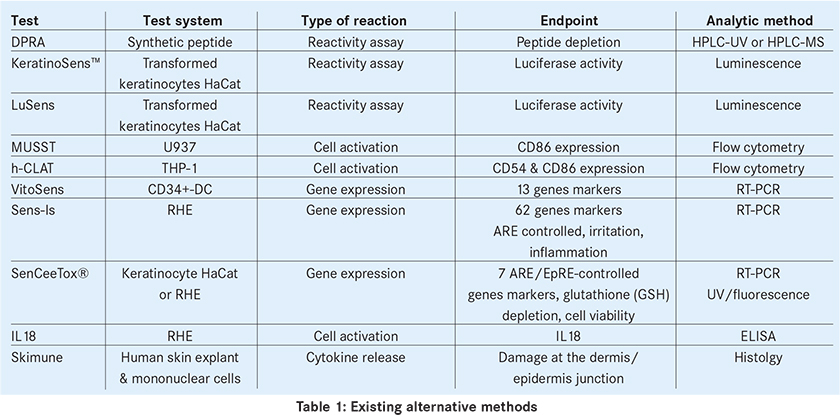
Validation is well advanced and even completed for some of these methods. This is the case for DPRA and KeratinoSens that have been subject of a positive peer review consensus report from ESAC (ECVAM Scientific Advisory Committee) and of recent EURL ECVAM recommendations18,19, and OECD has already issued a draft for a testing guideline for both methods. Validation is still ongoing for h-CLAT and MUSST and their completion is expected shortly.
The interest of cosmetic companies for these methods increases rapidly and the 4 pre-cited tests are currently the more widely used and available from CROs.
The main tests
DPRA
The DPRA was developed by Procter and Gamble in collaboration with the Strasbourg University in France in the laboratory of Pr Lepoittevin10. The test target the first step of the sensitisation phenomenon: the binding of the sensitiser on the skin proteins (covalent attachment of electrophilic substances to nucleophilic centres of skin proteins). It consists in measuring the binding capacity sensitising substance on two synthetic cysteine and lysine containing peptides (the thiol and amines groups of these amino acids are nucleophilic sites). The higher the affinity of the test substance to the peptide, the stronger will be the sensitising substance.
The test addresses one of the events placed at the macro-molecular interaction level of the Skin Sensitisation AOP.
The test measures by HPLC the residual peptide concentration after 24 hours of contact with the test element.
The accuracy of the DPRA for distinguishing sensitisers from non-sensitisers is 82% (76% sensitivity, 92% specificity)18.
This test has anyway some limitations. As it is an in tubo cell-free test it does not detect pro-haptens neither pre-haptens. A modified version of DPRA was therefore developed using the horseradish peroxidase-hydrogen peroxyde for the enzymatic activation of pro-hapten sensitisers20. As many in vitro methods, but more specifically, this test is not suitable for insoluble samples. Co-elution of the peptide with some of the sample compounds may also occur. This is especially true for complex samples such as vegetal extracts for which the interpretation if quite difficult. This can be overcome using mass-spectrometry detection. In addition some samples can react with the peptide with amino-acid other than cysteines or lysines, or can generate cysteines dimers by oxidation of the thiol groups. This can lead in a false positive result.
KeratinoSens
KeratinoSens has been developed by Dr Andreas Natsch form Givaudan11.
The test consists in evaluating the activation of AKR1C2 in transformed keratinocytes: KeratinoSens, by monitoring the induction of the luciferase gene fused to AKR1C2.
AKR1C2 gene is identified as a target gene for detecting skin sensitisers in dendritic cells. It is under the ARE control, that is activated by the protein Nrf2 via Keap1. The luciferase produced by the cells complexes with luciferin which, in the presence of ATP, produces light measured in relative light units (RLU).
A potential sensitiser links to Keap1 that releases Nrf2. Free Nrf2 is then capable to activate ARE and subsequently AKR1C2.
Even if it takes place in cells, the test is considered to also address one of the events placed at the macro-molecular interaction level of the Skin Sensitisation AOP.
The accuracy of the KeratinoSens for distinguishing sensitisers from non-sensitisers was reported as 85.1% (sensitivity 86.4%, specificity 82.6%) with respect to in vivo data for a set of 67 chemicals tested in house19.
The same limitations as for the DPRA can be seen for the KeratinoSens: the test method does not detect pro-haptens neither pre-haptens and is not suitable for insoluble samples. It has been also reported that the method has poor performance on weak sensitisers and it is likely to miss the few lysine reactive chemicals.
MUSST and h-CLAT
These tests are based on the activation of denditric cells. MUSST12 was developed by L’Oreal and is using the human myeloid U937 cells (Myeloid U937 Skin Sensitisation). The human Cell Line Activation Test (h-CLAT) was developed by Kao & Shiseido13 and is using the THP-1 cell line. Both tests are based on the enhancement by sensitisers of CD86 and/or CD54 expression on the denditric cells following exposure to an allergen (CD86 for MUSST and CD86 and/or CD54 for h-CLAT). They are therefore addressing one of the biological mechanisms covered by key event placed at the cellular response level of the skin sensitisation AOP.
Both tests entered in a formal validation phase. For h-CLAT, the EURL ECVAM coordinated validation study, performed in collaboration with the Japanese Center for the Validation of Alternative Methods (JaCVAM), has been completed and the ESAC peer review is currently on-going.
The Integrated Testing Strategy
As previously discussed, due to a mechanistic complexity of the final endpoint and the fact that no specific step or reaction has been identified as a key event for the determination of the potency of an allergen, an Integrated Testing Strategy (ITS) is needed for the full replacement of animal testing for skin sensitisation hazard identification and classification.
ITS approach will have to use a combination of individual alternative assays such as in silico, in chemico and in vitro methods and will certainly take into account the validated (or ongoing) methods that are already in use by cosmetic industries and CROs.
As animal testing is definitively not allowed anymore, cosmetic industries cannot afford to wait for an ITS and already use in vitro alternative methods as soon as they come out, whatever they are validated or not. As discussed the most used are currently the DPRA, the KeratinoSens (or LuSens), the h-CLAT and the MUSST. Based on the predictivity and the technical availability, as a testing strategy, the approach often used is as follow: 3 tests, i.e. DPRA, KeratinoSens or LuSens and h-CLAT or MUSST are performed together in combination and 2 of 3 tests must be matching for rating, e.g. to predict skin sensitisers 2 of 3 tests must be positive.
One can expect that more method will be available and validated in the near future and mainly that EURL ECVAM will take the leadership for the development of the skin sensitisation ITS and for its implementation in EU legislation8 in order to help the manufacturers in safety evaluation and classification.
AUTHOR
Frederic NUNZI, Doctor in Cell Biology and Microbiology
Head of IDEA Lab
email: f.nunzi@groupeideatests.com
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products.
- SCCS note of Guidance for the testing of cosmetic substances and their safety evaluation, 8th edition, 11 December 2012.
- Council Regulation (EC) No 440/2008 of 30 May 2008 laying down test methods pursuant to Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH)
- Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labeling and packaging of substances and mixtures
- OECD Guideline for Testing of Chemicals n° 406 – Skin Sensitisation – 17 July 1992.
- OECD Guideline for Testing of Chemicals n° 429 – Skin Sensitisation: Local Lymph Node Assay – 22 July 2010.
- Communication from the Commission to the European Parliament and the Council on the animal testing and marketing ban on the state of play in relation to the alternative methods in the field of cosmetics. COM(2013) 135 final, 11.3.2013.
- Casati, S. et al., (2013) EURL ECVAM strategy for replacement of animal testing for skin sensitisation hazard identification and classification. JCR Scientific and Policy Report.
- McKay, C. et al., (2013) From pathways to people : applying the Adverse Outcome Pathway (AOP) for skin sensitisation to risk assessment. Altex 30, p473-486.
- Gerberick, G.F. et al., (2004) Development of a peptide reactivity assay for screening contact allergens. Toxicol. Sci., 81, p332-343.
- Natsch, A., (2010) The Nrf2-Keap1-ARE toxicity pathway as a cellular sensor for skin sensitizers—functional relevance and a hypothesis on innate reactions to skin sensitizers. Toxicol. Sciences, 113(2), 284-292.
- Sakaguchi, H. et al., (2007) The in vitro skin sensitization test; human cell line activation test (h-CLAT) using THP-1 cells. Toxicology letters, 172, S93.
- Hooyberghs, J. et al., (2008) A cell-based in vitro alternative to identify skin sensitizers by gene expression. Toxicol. Appl. Pharmacol., 231(1), p103-111.
- Tessier, S. et al., (2012) SENS-IS®, an Episkin Based Model for Identifying Chemical Sensitizers. SOT's 51st Annual Meeting, March 11–15, 2012, San Francisco.
- McKim JM et al., (2010) A new in vitro method for identifying chemical sensitisers combining peptide binding with ARE/EpRE-mediated gene expression in human skin cells. Cutan. Ocul. Toxicol., 29(3), p171-192.
- Corsini E. et al., (2009) Use of IL-18 production in a human keratinocyte cell line to discriminate contact sensitizers from irritants and low molecular weight respiratory allergens. Toxicol. In Vitro., 23(5), p789-96.
- EURL ECVAM recommendationon on the Direct Peptide Reactivity Assay (DPRA) for skin sensitization testin. JCR Scientific and Policy Report – November 2013
- EURL ECVAM recommendationon on the KeratinoSens assay for skin sensitization testing. JCR Scientific and Policy Report – February 2014
- Gerberick, G.F., et al., (2009) Ivestigation of peptide reactivity of pro-hapten skin sensitizers using a peroxidase-peroxide oxidation system. Toxicol. Sci., 112, p164-174.



