Shisiedo has made its second recall in the space of a week, this time concerning three of its sunscreen products.
Sun medic UV medicated sun protect, Anessa whitening essence facial UV aqua booster and an Anessa limited-edition set have been voluntarily recalled.
The products, manufactured at the company's Osaka factory, are said to develop a granular texture, which feels uncomfortable when applied to the skin.
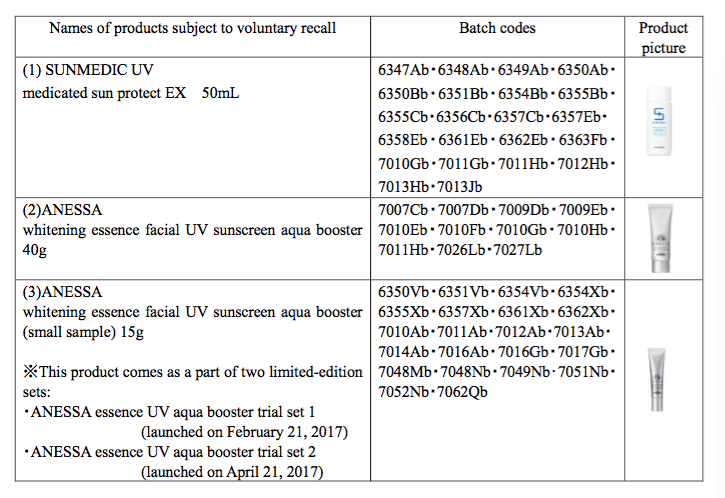
Shiseido said the three products do not meet its quality standards, although reassured the public that they still protect against UV damage and do not pose a safety risk.
It told Cosmetics Business: "We, at Shiseido, comply with any applicable laws and regulations, and place importance on offering high-quality and ultimately safe products that would satisfy consumers based on our rigid internal regulations.
"Following those recalls, we have started revising the internal systems from various perspectives and will place further emphasis on quality control to prevent any reoccurrence.”
The recall comes just six days after Shiseido Group recalled a number of its body washes, manufactured in its Kuki Factory between January and July. However Shiseido confirmed the recalls are unrelated.
The body washes had an ‘uncharacteristic odour’, which Shiseido said did not meet its standards.

