Author: Dr. P. May, Flavex Naturextrakte GmbH, Rehlingen, Germany
Abstract
Supercritical CO2-extraction is the state-of-the-art technology for the production of standardised Arnica-extracts with high concentration of bioactive sesquiterpene lactones (SQLs). This provides the base for the development of valuable dermatological products with good efficacy, whilst simultaneously minimising the allergenic potential. A further advantage is that the lipophilic CO2-extract does not contain polar components e.g. flavonoids which are suspected of allergenic effects.
Traditional use of Arnica preparations is supported by new pharmacological and clinical studies. Besides the application in the case of sports and accident related injuries, Arnica is increasingly used for treatment of age-related inflammatory diseases of the bones and joint apparatus. Topical treatment with Arnica preparations can be ranked today among rational phytotherapy. Arnica should not be administered orally or applied to open wounds where absorption can occur. The predominant opinion in literature that Arnica SQLs have strong allergenic potential could not be confirmed in recent investigations. Highly concentrated plant extracts standardised to bioactive ingredients have an enormous potential in skin care and dermatology when used properly.
Introduction
Knowledge about the healing power of Arnica can be traced back to the 13th century(1). Arnica preparations have traditionally been used for the treatment of backache, bruising, cramps, fibrositis, relief of muscular and rheumatic pain and stiffness, sport injuries and sprains.

Figure 1(b) Arnica Montana
Recent clinical studies for topical treatment of inflammatory diseases with Arnica preparations support the traditional use of Arnica for a variety of injuries and muscle or joint pains. The genus Arnica belongs to the Asteraceae family and comprises about 32 species which which are divided into five subspecies: andropurpurea, arctica, austromontana, chamonissonis and montana. The European Pharmacopoeia monograph specifies only Arnica montana. The height of Arnica montana ranges from 30-60cm. It is a perennial plant with a round, hairy stem and one to three flower stalks. Flowers are yellow- orange ray flowers around a cluster of tubular center flowers. The distribution area of Arnica montana is mainly the alpine Mediterranean region from the Pyrenees in the North of Spain to the Balkans, from the valleys up to 2500m altitude.
The main ingredients of Arnica montana flowers are SQLs of the pseudoguaianolide type 0.3-0.9%(2), flavonoids 0.4-06%(3), essential oil 0.23-0.35%(2), polyacetylene(4), polysaccharides(5) and triterpenes(6).
The SQL composition of different origins of Arnica varies considerably even if the total concentration is relatively constant(7,8). Two chemotypes of Arnica montana are identified. The helenalin type is native to Central Europe and the dihydrohelenalin type, which contains only traces of helenalin, is native to Spain. SQLs consist mainly of esters of helenalin and 11α,13-dihydrohelenalin with short-chain fatty acids such as acetic, isobutyric, isovaleric, methacrylic and tiglic acids(9). These compounds are known as helenanolides, i.e. pseudoguaian-7β,8β-olides.
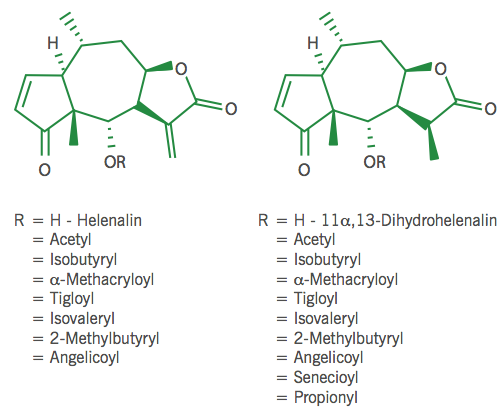
Figure 2 Helenalin and dihydrohelenalin and their esters
CO2-Extraction and Analysis
Arnica has been wild crafted for centuries. Due to the health benefits, the demand has been growing for decades, which leads to over-harvesting in traditional collection areas. For that reason Arnica montana has been included in the Washington Convention of Endangered Species (CITES). Arnica montana is also listed in Annex D of the EU Council Regulation No. 338/97 which implements CITES. The collection is therefore controlled in many countries. For extraction our company uses only high quality flower heads of Arnica montana from sustainable collections and a cultivation project has been started.
CO2-extraction is the best technology for the extraction of Arnica flowers, since it works under gentle conditions without temperature stress and the influence of oxygen. Supercritical CO2-extracts completely preserve the lipophilic plant ingredients, they are highly
concentrated and do not contain any solvent residues. Esterified SQLs cannot be obtained by steam distillation. Furthermore, the SQLs are concentrated many times higher in the CO2-extract than in alcoholic extracts. The genuine Arnica flower CO2-extract is standardised by the addition of sunflower oil to 4% SQLs so that it can be easily dosed. The supercritical CO2-extract is unique in terms of concentration and composition of bioactive ingredients (see Table 1). It is also available in EU-organic quality and USDA-NOP quality and meets the strict criteria of natural cosmetic standards e.g. BDIH, COSMOS, ICADA, NATRUE, NPA, NSF ANSI 305.
SQLs are generally analysed by GC or HPLC(10). Comparative quantitative analysis with HPLC, GC and spectrophotometry showed similar test results(11). Wagner developed and validated a GC-MS method for separation and quantitative analysis of the SQLs from Arnica flowers(12). Helenalin and 11α,13- dihydrohelenalin derivatives are detected in the SIM mode by their specific molecular masses. TLC is the preferred method for identity control and visualisation of constituents. The standardised Arnica flower CO2-extract is specified as follows:
Standardised Arnica Flower CO2-extract
| Raw Material | Arnica montana - Flowers |
| Origin | Spain, Romania |
| Sum of Helenalin and Dihydrohelenalin Esters | 4% |
| Triterpenediol Esters | 2.0-4.5% |
| Helenalin and Dihydrohelenalin | ~0.1% |
| Content of Essential Oil | 0.2-0.4% |
Table 1. The Standardised Arnica Flower CO2-extract
Pharmacology
In general Arnica is applied topically with good efficacy for symptomatic treatment of complaints connected with inflammation. In order to achieve anti-inflammatory efficacy, the active ingredients have to penetrate and subsequently permeate the skin. It has been demonstrated that the SQLs of different Arnica preparations show a comparable penetration in and permeation through the stratum corneum. The permeated concentration is sufficient for anti-inflammatory efficacy. It is interesting that isolated SQLs have a significantly poorer penetration performance than the SQLs of an Arnica extract and dihydrohelenalin and its derivatives have a better penetration than helenalin derivatives. A gel preparation showed a decrease of the penetration rate over time, whereas the penetration rate of ointments remained constant over time(14,15).
A literature review reveals that the SQLs of Arnica montana have a broad spectrum of effects when applied topically:
- analgetic
- anti-arthritic
- antibacterial
- anticancer
- anti-inflammatory
- antimycotic
- antiplatelet.
These indications are demonstrated in vitro(16,17,18,19,20) and in vivo(21,22,23,24) and they correlate closely with the therapeutic areas of Arnica flower preparations(25).
Arnica preparations have been used topically for centuries for the treatment of injuries, sprains, bruises and haematomas(26,27,28,29). In recent decades, pharmacological investigations with SQLs of Arnica have confirmed their effectiveness. The latest research in the field of chronic and age-related diseases proves the good efficacy e.g. in the case of rheumatic and inflammatory conditions such as osteoarthritis(30,31) and chronic venous insufficiency(32,33,34).
Osteoarthritis comprises a group of musculo-skeletal disorders. It is a chronic degenerative disease of the joints, especially of the hips, knees and fingers which can lead to degradation of joint cartilage and subchondral bone. This disease is prevalent in an ageing society. Symptoms of osteoarthritis are pain, stiffness, joint deformities and reduced mobility. Osteoarthritis is typically treated with analgesics as well as with non-steroidal antiphlogistics.
The efficacy of an Arnica montana gel was investigated in an open multicentre study with 53 women and 26 men with mild to moderate osteoarthritis of the knee. The gel was applied twice daily and after two weeks the symptom relief was comparable to topical treatment with a Diclofenac-gel(30). After three and six weeks significant improvement was demonstrated.
In another randomised, double-blind study with 204 patients who suffered from osteoarthritis of the hands, an Arnica gel was tested in comparison to a 5% ibuprofen gel. The gel was rubbed into the affected wrists three times a day. Pain relief and hand function were investigated after 21 days of treatment. Both preparations led to equally good results regarding pain relief and functional improvement of the hand(31).
Arnica has a strong anti-inflammatory and blood circulation- enhancing effect and thus it is very well suited for treatment of chronic venous insufficiency (CVI). Several clinical studies with Arnica gels demonstrate the significant improvement of symptoms and venous backflow as well as the decrease in venous capacity(32,33,34).
In a prospective open multi-centre observational study with 148 patients, the efficacy of a topical Arnica preparation for treatment of sub-acute ankle joint injury resulting from contusion and distortion could be confirmed. During a two-week period of observation efficacy was approved. Swelling of the ankle joint was reduced to normal compared with the ‘healthy side’(35).
In comparison with a placebo, an Arnica gel also showed good efficacy in the treatment of 12 patients with muscle soreness. After a treatment period of six days, symptoms reduced and no side effects or intolerability were observed(36).
The European Commission has approved the external use of Arnica flower for injuries and for the consequences of accidents, e.g. sprains, haematoma, dislocations, contusions, oedema due to fracture, rheumatic muscle and joint problems. It is also approved for the treatment of superficial phlebitis and inflammation caused by insect bites. In cases of inflammation, Arnica preparations also show analgesic and antiseptic activity. In animal studies, helenalin and dihydrohelenalin and their esters were found to have analgesic, antibiotic, antimicrobial and anti-inflammatory activity(16,23).
Mechanism of Activity

Figure 1(c) Arnica Montana
The anti-inflammatory activity of Arnica extracts applied topically has been proven in a range of in vitro studies on isolated enzymes and cellular systems. The concentration of SQLs is responsible for the pharmacological activity. Both helenalin and dihydrohelenalin and their esters already act in very low concentrations in the biochemical pathways of the cells by inhibiting the transcription factor NF-κB, a central mediator of the human immune response system. Helenalin not only inhibits the NF-κB activation but also decreases the production of many inflammatory cytokines. This prevents the recruitment of immune cells, T- and B-cells, as well as macrophages and neutrophils and thus helenalin reduces inflammation. It has been shown that helenalin can inactivate previously activated NF-κB, a decisive factor for treatment of inflammation(37).
Helenalin binds directly to thiol (sulfhydryl) groups on the amino acid cysteine in NF-κB, thus blocking its transcription activities(38). Both the α-methylene-γ-lactone and α,β- unsaturated cyclopentenone structures in helenalin are involved in this mechanism, which explains why 11α,13-dihydrohelenalin lacking the α-methylene group, is less active and inhibits NF-κB only at higher concentrations than helenalin(38,39).
New Evidence on Allergenic Potential
The allergenic potential is a difficult subject since many factors are involved in an allergic response. The Arnica species used in a formulation, the type of extract, the concentration of active ingredients, other ingredients in the formulation and, of course, the applied test model for sensitisation all have influence on the test results.
In literature the allergenic potential of Arnica has been discussed controversially in the past. Some time ago the allergenic potential of Arnica was investigated using the guinea pig skin erythema test with the result that it was classified as a strong contact allergen(40).
However the disadvantages of this test model are obvious e.g. choice of test concentration, choice of vehicle, practice of reading, use of control animals and interpretation of test results vary widely and are not standardised. This could lead to overestimation of the sensitisation hazard for certain substances(41, 42).
A new in vitro and in vivo study at the department of allergology at the renowned dermatological clinic of Freiburg demonstrates that topically applied Arnica preparations are not strong contact allergens(43). The investigations were performed by using the mouse contact hypersensitivity model, which is today the standard model for allergic contact dermatitis assessment(44,45). The anti-inflammatory and allergenic potential of alcoholic Arnica tinctures from Arnica montana Spanish type and Central European type were investigated beside the effect of pure helenalin and 11α,13-dihydrohelenalin(43). It was found that Arnica tinctures as well as helenalin or dihydrohelenalin are only weak contact sensitisers. Another study which is in accordance with these results demonstrates that the SQLs of helenalin and dihydrohelenalin type did not play a major role in positive allergenic tests with Arnica. There was also no difference found between helenalin and dihydrohelenalin type of SQLs regarding allergenic reaction(46,47).
Further investigations, however, reveal that the SQLs have a dual effect: at high concentration they are anti-inflammatory by inhibiting NF- κB DNA binding through alkylation of NF- κB p65(38,48) and at low concentration they have a pro-inflammatory and pro-allergic effect as a result of NF- κB activation and hapten modification of proteins to generate antigenic T cell determinants(49,50). In vitro tests with human keratinocytes and dendritic cells of mouse and human have shown that the balance between these opposing effects is dose dependent. The standardised Arnica CO2-extracts with a high concentration of SQLs are therefore the first choice for the development of products with good efficacy and control over allergenic reaction.
The anti-inflammatory activity of dihydrohelenalin is lower than that of helenalin. Therefore the concentration of dihydrohelenalin derivatives must be approximately 10-fold higher than the concentration of helenalin derivatives, in order to achieve the same anti-inflammatory effect. In the mouse model contact hypersensitivity could not be induced even if the Arnica tinctures and SQLs are applied undiluted to inflamed skin.
Authors Note - Dr. Peter May studied chemistry at the University of Saarland and received his Ph.D. in 1991 in metal-organic chemistry. Dr. May has been the author of articles on plant science and phytotherapy in several publications. Flavex is a producer of specialty botanical extracts for cosmetics and for the food supplement industry on the basis of supercritical CO2-extraction.
The findings above are supported by recent clinical studies. In spite of extensive use of Arnica preparations, the frequency of allergy occurrence is surprisingly low, if used properly. A proper use requires analytical, well documented extracts and formulations with defined and standardised composition of active substances. Furthermore, indication of dosage ranges is crucial for achieving good efficacy and controlling allergenic response. This is confirmed by several recent studies. Only three volunteers out of 213 who reacted positively to the composite mix (Arnica, Camomile, Feverfew, Tansy and Yarrow) showed a positive reaction to a 0.5% ether extract of Arnica(51). In another study with the same setting, only five of 443 volunteers showed a positive reaction(52). In a European multi-centre study on allergic contact dermatitis, only one volunteer out of 475 developed an allergy(53).
Contact dermatitis of Arnica preparations described so far were performed almost without exception with Arnica tinctures whereby it was not always clear if they were used in the correct dilution (54). These statements are in accordance with the results of a Danish study demonstrating that sensitisation occurred mainly from the topical use of Arnica tinctures which had highly variable SQL concentrations(55). In the tinctures and infusions SQLs were partly detected in a very low concentration or only in traces whereas other analysed samples, e.g. an Arnica CO2-extract was much higher in SQLs(56). It seems therefore reasonable to suppose that such low concentrated SQL formulations can cause allergy due to the dual effect of SQLs. It also suggests that the presence of other allergens in low SQL infusions and tinctures which elicited positive allergenic reactions(56). Also ether extracts contain not only SQLs, but also other potential allergens(51). Arnica should not be taken internally, except in the form of a homeopathic dilution. Arnica should not be applied to injured skin where absorption can occur.
Conclusion
Supercritical Arnica extracts are the first choice for the development of high quality dermatological products. They are analytically well documented and contain a high and standardised concentration of the active SQLs while simultaneously the allergenic potential is under control.
1. Freyer M: Europäische Heilkräuterkunde. Ein Erfahrungsschatz
aus Jahrtausenden (Würzburger medizinhistorische Forschungen, 61).
Königshausen&Neumann, Würzburg: S. 37., 1998
2. Willuhn G: Arnika Flowers: Pharmacology, toxicology and analysis of
the sesquiterpene lactones – their main active substances. In: Lawson
LD and Bauer R, editors. Phytomedicines of Europe – chemistry and
biological activity. ACS Symposium Series 691. Washington DC: American
Chemical Society:118-132, 1998
3. Willuhn G, Merfort I, Paßreiter CM, Schmidt TJ: Chemistry and
systematics of the genus Arnica. In: Hind, DJN, Jeffrey C, Pope
GW
(Hrsg.): Advances in Compositae Systematics. Royal Botanic Gardens,
Kew, England:167-195, 1995
4. Schulte KE, Rücker G, Reithmayr K: Einige Inhaltsstoffe von Arnica
chamissonis und anderer Arnica-Arten. Lloydia32:360-368, 1969
5. Wagner H, Proksch A, Riess-Maurer I, Vollmar A, Odenthal S, Stuppner
H et al: Immunstimmulierend wirkende Polysaccharide (Heteroglykane) aus
höheren Pflanzen. Arzneim.-Forsch./Drug Res. 35: 1069-1075, 1985
6. Schmidt TJ, von Raison J, Willuhn G: New triterpene esters from
flowerheads of Arnica lonchophylla. Planta Med. Oct;70(10):967-
77, 2004
7. Perry NB, Burgess EJ, Rodríguez Guitián MA, Romero FR, López
Mosquera E, Smallfield BM, Joyce NI and Littlejohn RP: Sesquiterpene
lactones in Arnica montana: helenalin and dihydrohelenalin
chemotypes
in spain. Planta Med 75: 660-666, 2009
8. Pharmacopoiea Europaea 6. Ausgabe, 3. Nachtrag, 5365 ff, 2009
9. Willuhn G, Röttger P-M, Matthiesen U: Helenalin and
11,13-dihydrohelenalin
esters from flowers of Arnica montana. Planta Medica 49:
226-231, 1983
10. Merfort, I: Review of the analytical techniques for sesquiterpenes
and
sesquiterpene lactones. J. Chromatogr.A 967(1): 115-13-, 2002
11. WIlluhn G. & Leven W: Zur qualitativen und quantitativen
Analyse der Sesquiterpenlactone von Arnikablüten DAB 9. Pharm. Ztg.
Wiss., 1(136): 32-30, 1991
12. Wagner, S: Sesquiterpenlactone: Neuronale Netze als QSAR Modell
sowie pharmakokinetische Untersuchungen am Beispiel von Arnica
montana.
Dissertation, Albert Ludwigs-Universität, Freiburg im Breisgau, 2006
13. Isaac O: Die Ringelblume, Botanik, Chemie, Pharmakologie,
Toxikologie, Pharmazie und therapeutische Verwendung. Wissenschaftliche
Verlagsgesellschaft mbH, Stuttgart S. 60, 1992
14. Wagner S, Suter A,Merfort I: Skin penetration studies of Arnica
preparations and of their sesquiterpene lactones. Planta Med. 70:
897-903, 2004
15. Wagner S, Merfort I: Skin penetration behaviour of sesquiterpene
lactones from different Arnica preparations using a validated
GC-MSD
method. J Pharm Biomed Anal 43: 32-38, 2007
16. Hall IH, Lee KH, Stames CO, Sumida Y, Wu RY, Waddell TG et al:
Anti- inflammatory activity of sesquiterpene lactones and related
compounds. J. Pharm. Sci 68: 537-542, 1979
17. Woerdenbag HI, Merfort I, Paßreiter CM, Schmidt TJ, Willuhn G, van
Uden W et. al: Cytotoxicity of flavonoids and sesquiterpene lactones
from Arnica Species against GLC and the COLO 320 Cell lines.
Plantamedica 60: 434- 437, 1994
18. Lee KH, Ibuka T, Wu RY, Geissman TA. Structural antimicrobial
activity relationships among the sesquiterpene lactones and related
compounds. Phytochemistry: 16: 1177-1181, 1977
19. Willuhn G, Röttger PM, Quack W: Untersuchungen zur
antimikrobiellen Aktivität der Sesquiterpenlactone der Arnikablüten.
Pharm Ztg. 127: 2183- 2185, 1982
20. Schröder H, Lösche W, Strobach H, Leven W, Willuhn G, Till U,
Schrör K:Helenalin and 11 alpha,13-dihydrohelenalin, two constituents
from Arnica montana L., inhibit human platelet function via
thiol-dependent pathways. Thrombosis Res. 57:839-845, 1990
21. Mascolo N, Autore G, Capasso F et al: Biological screening of
Italian medicinal plants for anti-inflammatory activity. Phytotherapy
Research 1(1):28-31, 1987
22. Hall IH, Starnes CO, Lee, KH, Waddell TG: Mode of action of
sesquiterpene lactones as anti-inflammatory agents. J. Pharm. Sci.
69:537-543, 1980
23. Klaas CA, Wagner G, Laufer S, Sosa S, Della Loggia R, Bomme U et.
al: Studies on the anti-inflammatory activity of phytopharmaceuticals
prepared from Arnica flowers. Planta Medica 68:385-391, 2002
24. Hall IH, Lee KH, Starnes CO, Eigebaly SA, Ibuka T, Wu YS et al:
Antitumor agents XXX: evaluation of α-methylene-γ-lactone-containing
agents for inhibition of tumor growth, respiration and nucleic acid
synthesis. J. Pharm. Sci. 67:1235-1239, 1978
25. von Raison J, Heilmann J, Merfort I, Schmidt TJ, Brock FE, Leven W,
Bomme U, Bauer R: Arnika - Arzneipflanze mit Tradition und Zukunft,
Zeitschrift für Phytotherapie 21:39-54, 2000
26. Hänsel R, Keller K, Rimpler H, Schneider (Hrsg.): Hagers Handbuch
der pharmazeutischen Praxis, Monographie: Arnica. Band 4:
Drogen A-D,
5. Aufl., Springer Verlag Berlin 342-357, 1994
27. Kommission E: Monographie: Arnicae flos (Arnikablüten).
BAnz Nr.
228 vom 05.12.1984
28. Madaus G.: Arnica montana; in: Lehrbuch der biologischen
Heilmittel
Band 3, Nachdruck der Ausgabe Leipzig 1938, Mediamed Ravensburg
585-596, 1987
29. Meyer-Chlond G: Arnika-Arzneipflanze mit Tradition und Zukunft. DAZ
34: 87-90, 1999
30. Knuesel O, Weber M, Suter A. Arnica montana gel in
osteoarthritis
of the knee: an open, multicentre clinical trial. Advances in Therapy
19:209- 218, 2002
31. Widrig R, Suter A, Saller R, Melzer J: Choosing between NSAID and
Arnica for topical treatment of hand osteoarthritis in a
randomised,
double-blind study. Rheumatol Internat 27:585-591, 2007
32. Uehlecke B. Local application of Arnica in patients with
chronic
venous insufficiency due to venous varicosis. (Abstract) 3rd annual
symposium on complementary health care. ForschKomplementärmed 3: 327,
1996
33. Brock FE: Ergebnisse klinischer Studien bei Patienten mit chronisch
venöser Insuffizienz. Z. Phytother. 21: 49-50, 2000
34. Brock FE: Additiver Effekt venentypischer Hydrotherapie nach Kneipp
und lokaler Arnika-Anwendung bei Patienten mit chronisch venöser
Insuffizienz. 50:357-363, 2001
35. Dr. med. Dr. rer. nat Michael Kroll, Dr. Josef K. Merges, PD Dr.
med. Gerhard Wolf, MIT GesundheitGmbH, Flutstr. 74, 47533 Kleve
36. Moog-Schulze JB. Eey/-3-n medischexperimenteelonderzoeknaar de
werkzaamheid van eenuitwendigetoepassing van Arnica-gelei. TIG
9(3):105-112: 1993
37. Lyß G, Schmidt TJ, Merfort I, Pahl HL: Helenalin, an
anti-inflammatory sesquiterpene lactone from Arnica selectively
inhibits transcription factor NF-κB. Biol. Chem. 378: 951-961, 1997
38. Lyß G, Knorre A, Schmidt TJ, Pahl HL, Merfort I: The
anti-inflammatory sesquiterpene lactone helenalin inhibits the
transcription factor NF-κB by directly targeting. p65. J. Biol. Chem.
273:33508-33516, 1998
39. Rüngeler P, Castro V, Mora G, Gören N, Vichnewski W, Pahl HL et
al: Inhibition of transcription factor NF-κB by sesquiterpene lactones:
a proposed molecular mechanism of action. Bioorg. Med. Chem. 7:2343-
2352, 1999
40. Hausen BM: Arnica allergy. Hautarzt31:10-17, 1980
41.
http://ec.europa.eu/health/scientific_committees/consumer_safety/opinions/
sccnfp_opinions_97_04/sccp_out102_en.htm
42. Basketter DA, Andersen KE, Liden C et al: Evaluation of the skin
sensitising potency of chemicals by using the existing methods and
considerations of relevance for elicitation. Contact Dermatitis 52:
39-43, 2005
43. Lass C, Vocanson M, Wagner S, Schempp CM, Nicolas JF, Merfort I,
Martin SF: Anti-inflammatory and immune regulatory mechanisms prevent
contact hypersensitivity to Arnica Montana. L. Exp.Dermatol.
17(10):
849-857, 2008
44. Basketter DA, Andersen KE, Liden C et al: Evaluation of the skin
sensitising potency of chemicals by using the existing methods and
considerations of relevance for elicitation. Contact Dermatitis 52:
39–43, 2005
45. Basketter DA, Smith Pease CK, Patlewicz GY: Contact allergy: the
local lymph node assay for the prediction of hazard and risk.
ClinExpDermatol 28: 218–221, 2003
46. Jocher A, Nist G, Weiss JM, Wetzel D, Merfort I, Jakob T, Schempp
CM: Allergenic potential of Arnica-containing formulations in
Arnica-allergic patients. Contact Dermatitis 61: 304–306, 2009
47.
MerfortI.:Arnika-aktuellerStandhinsichtlichWirksamkeit,Pharmakokinetik
und Nebenwirkungen, Zeitschrift für Phytotherapie 31: 188-182, 2010
48. Garcia-Pineres AJ, Castro C, Mora G et al: Cysteine 38 in p65
⁄NF-kappa B plays a crucial role in DNA binding inhibition by
sesquiterpene lactones. J Biol Chem 276: 39713–39720, 2001
49. Martin SF: T-lymphocyte mediated immune responses to chemical
haptens and metal ions – implications for allergic and autoimmune
disease. Int Arch Allergy Immunol 134: 186–198, 2004
50. Saint-Mezard P, Berard F, Dubois B, Kaiserlian D, Nicolas JF: The
role of CD4+ and CD8+ T cells in contact hypersensitivity and allergic
contact dermatitis. Eur J Dermatol 14: 131–138, 2004
51. Geier J, Hausen BM:Epikutantestung mit dem Kompositen-Mix.
Allergologie 23: 334–341, 2000
52. Reider N, Komericki P, Hausen BM, Fritsch P, Aberer W: The seamy
side of natural medicines: contact sensitisation to Arnica (Arnica
montana L.) and marigold (Calendula officinalis L.). Contact
Dermatitis. Nov;45(5):269- 72, 2001
53. Paulsen E. Contact sensitization from Compositae-containing herbal
remedies and cosmetics. Contact Dermatitis 47: 189-198, 2002
54. Merfort I: Arnika. Neue Erkenntnisse über eine alte Arzneipflanze.
Internist. Prax. 47:369-376, 2007
55. Paulsen E, Andersen KE: Patch testing with constituents of
compositae mixes. Contact Dermatitis 66:241-246, 2012
56. Paulsen E, Christensen LP, Andersen KE. Cosmetics and herbal
remedies with compositae plant extracts - are they tolerated by
compositae-allergic patients? Contact Dermatitis 58:15-23, 2008

