What is the Nagoya Protocol?
Animals, plants and microorganisms provide millions of useful benefits already. This barely begins to scratch the surface of what might yet be achieved.
So how do we encourage the development of untapped resources, and more importantly how do we do it in a fair way that rewards both those researching the benefits and those who provide the raw materials?
Every country holds sovereign rights over the animals, plants and genetic resources, including microorganisms and traditional knowledge, found within its borders.
The United Nations Convention on Bio Diversity (Rio, 1992) was agreed by 172 countries and defined these rights by treaty.
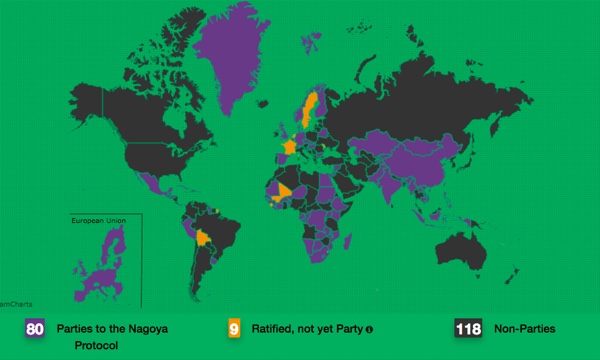
Many countries have now ratified the treaty and the Nagoya Protocol (Japan, 2010) deals with the third objective of the treaty, which refers to access and benefits sharing when exploiting genetic resources.
The ABSCH website is the one-stop shop for information and compliance on the Nagoya Protocol.
The Nagoya Protocol came in to force on 12th October 2014 after the 50th country ratified the protocol.
Each country that ratified the protocol has national government contacts who will record all activity of incoming requests and consents and enforce the protocol within its national borders.
The details of these contacts and national bodies, plus all activity under the protocol, is recorded on the Access and Benefit Sharing Clearing House (ABSCH), which is a publicly accessible internet information resource and the global one-stop shop for compliance: absch.cbd.int
This website will have a profile for each country with details of all agreements issued by that country.
Access and Benefit Sharing (ABS)
Access and benefits sharing is the main focus of the protocol.
This defines the mechanism by which individuals or organisations who wish to use genetic resources for research must both respect the rights of the provider country and obtain the correct permission to use those resources in their research.
Put simply, any organisations wishing to use certain materials or traditional knowledge for research and development purposes must, before any access to the materials takes place, come to an agreement on the benefits to be shared with the provider country.
Nagoya World
Prior Informed Consent (PIC)
The first step is obtaining 'Prior Informed Consent' from the country which owns the rights to the genetic materials.
The researcher or company must make a PIC request to the country. If the initial request is successful, they can move on to agreeing the terms.
Mutually Agreed Terms (MAT)
These are the contractual terms between the provider country and the organisation or individual requesting access to the genetic material or knowledge.
They will explain the benefits to the provider country, which may be financial or other benefits such as knowledge sharing and training.
These terms will also define the term of the agreement and conditions under which those benefits must be offered.
For example, a provider country may accept a percentage of the value generated by any marketed product or IP rights created as a result of the research.
They will also define under what conditions those rights and benefits can be sold on.
Checkpoints
Official checkpoints have to be implemented in countries where genetic resources are being utilised.
These checkpoints will verify whether PIC has been obtained and MAT established.
The official checkpoints allow the central database to be updated so the provider country can track the research and development of its genetic material.
Checkpoints can also be used by recipient countries where grants are issued to support research.
The grants will be subject to due diligence, provided by the organisation, which will show whether the right steps have been followed to ensure compliance with the Nagoya Protocol.
Scope
Human material is exempt from the protocol and regulated by different legislation.
Scope will help organisations understand whether their research will trigger compliance with the protocol.
It is only necessary to follow the protocol for material that is to be supplied from countries who are party to the Nagoya Protocol, and if the material is to be supplied after its enforcement on 12th October 2014.
Even for countries who are party to the Nagoya Protocol, they must have 'access legislation' in place.
The UK and many other European countries, for example, have decided not to put access measures in place for genetic materials found within their borders.
Spain and France, however, do have access measures in place so organisations should research each individual country to see what those measures are.
Of course, if other environmental legislation exists in the country then this must still be complied with regardless of the Nagoya Protocol.
Materials in scope of the Nagoya Protocol are animal, plant and microbial materials. It does not apply to human human genetic material.
The protocol also only applies to these materials when used in research and development, not for materials being used in formulation to manufacture finished products.
Certificates of Compliance (IRCC)
Internationally Recognised Certificates of Compliance are issued by the provider country when all the information has been supplied and terms agreed; in other words, when the PIC and MAT have been completed.
The certificate is then published on the Access and Benefits Sharing Clearing House website by the provider country.
European Union guidance
EU guidance for cosmetics is currently in the process of being produced.
The European Union is providing guidance for the Nagoya Protocol at two levels.
Initially it provides guidance at a horizontal level, which is general guidance applying to all industries.
The EU also plans to provide guidance for specific vertical sectors, and cosmetics and pharmaceuticals are two of those sectors.
The Cosmetics Sector guideline document was in the process of being developed at the time of writing this article.
A Due Diligence Declaration must be supplied to the national authority where the research takes place when EU grants are being applied for or when regulatory approval for a new product is given.
The national authority will then issue a checkpoint communique which is published on the ABSCH website.
Conclusion
It’s early days for the CBD and its child the Nagoya Protocol.
The protocol has not had much engagement so far, with only four countries issuing IRCCs on the ABSCH website.
India is currently leading the way, with mostly domestic agreements in place.
However, the development cycle in cosmetics, pharmaceuticals and food can take years.
Competitive advantage is key within these industries, and will also challenge the legislation to ensure commercial-in-confidence manoeuvres do not dilute the objectives of public compliance too far.
It will also be difficult to police research and development, although each national authority has an obligation to do this.
Within European academic institutions, the necessity of grant applications will ensure that the Nagoya Protocol is considered during the application process.
The United States of America is not party to the protocol.

At present, there are many countries who are not covered by the Nagoya protocol. Either they have not yet ratified the protocol or they have decided it is too much of a burden. Notably, the US has not ratified the CBD, as a result it cannot become party to the Nagoya Protocol.
However, it is still early days, and the developed world is looking to the undiscovered world more and more.
The Nagoya Protocol is therefore expected to have a big impact on the cosmetics, pharmaceutical and health care industries in the future.
