Oils, fats and waxes are important raw materials used throughout numerous industries. This essay offers an overview of the types and characteristics of oils, fats and waxes, outlining the difficulties in achieving a distinct classification.
Consideration is given to both natural and synthetic oils, fats and waxes, examining their origin, and the production and processing techniques applied to each.
Focusing on their role within the cosmetics industry, examples of their uses will be discussed, along with a review of the potential limitations and formulation problems that can arise as a result of their chemical nature.
What are oils, fats and waxes?
An oil is defined as “any of a group of natural esters of glycerol (glycerine) and various fatty acids, which are liquid at room temperature”. Fats are very similar to oils and are defined as “any of a group of natural esters of glycerol and various fatty acids, which are solid at room temperature and are the main constituents of animal and vegetable fat”.
The only discernible difference between fats and oils is their state at ambient temperature. Within cosmetics, waxes can be ascribed as a usually solid organic compound: harder, more brittle and with a higher melting point than fats. However, some natural waxes can be soft semi-solids or even liquid.
Figure 1:
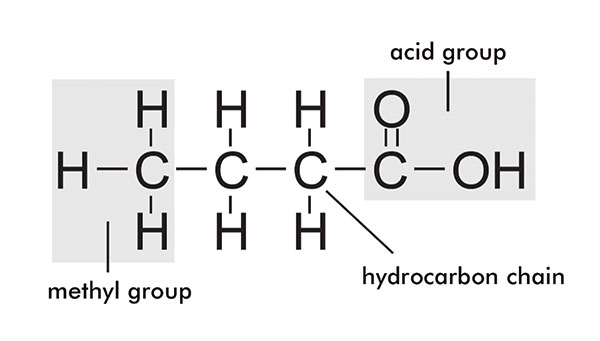
Fatty acid structure
Although different, oils, fats and waxes can be subdivided into the same classifications, ie natural, comprising ‘true’ and natural derivatives, and synthetic, comprising petrochemical derivatives and silicones.
Natural oils, fats and waxes
True oils, fats & waxes: Origin & structure
Natural oils, fats and waxes are primarily obtained from either plant or animal sources, including sunflower, oilseed rape, oil palm, beef tallow, lanolin and beeswax. However, in recent years, algae oil has made an appearance as an attractive alternative for use in cosmetic and toiletry products.
Oils and fats are organic compounds comprised of esters of glycerine and fatty acids. Glycerine is a trihydric alcohol which forms triesters with the fatty acids. The fatty acids comprise a straight carbon backbone (usually with an even number of carbon atoms) and a carboxyl group at one end.
The carbon chains can be saturated or unsaturated and vary in length from six to 22. The diagram in figure 1 shows the structure of fatty acids. Glycerine and fatty acids together form triglycerides and this is presented in figure 2.
Figure 2:
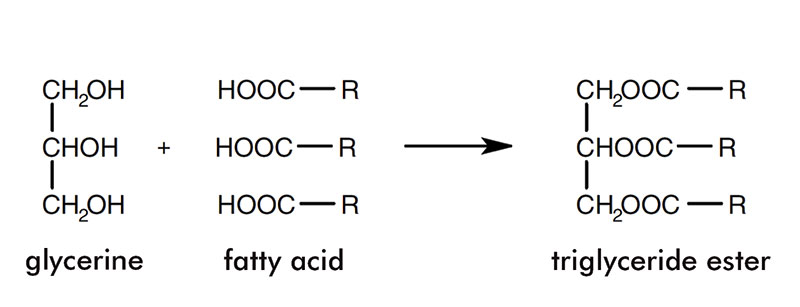
Triglycerides; R = a chain of between six and 22 carbon atoms
The different types of triglyceride present give oils and fats their various properties. For example, whether the triglycerides are saturated or unsaturated affects the melting point, unsaturated triglycerides having a lower melting point than saturated triglycerides with the same carbon chain length.
The triglycerides’ carbon chain length also affects the melting point, with a longer chain giving a higher melting point. Waxes are also comprised of esters. However, they mainly consist of monoesters, which are formed between a fatty alcohol molecule and a fatty acid molecule.
These monoesters range in chain length, from 16-30 carbon atoms, and are usually saturated.
The only discernible difference between fats and oils is their state at ambient temperature
True oils, fats & waxes: Production
Plant oils & fats production
Plant/vegetable oils and fats can be extracted from any oil-bearing part of the plant, usually from the seed, nuts or fruit. Essentially, the same method of extraction is used for the majority of vegetable oils and fats.
This involves a process of pressing, cooking and solvent extraction. The plant material is cleaned and, if large and/or un-uniform in size, ground to give a larger surface area for more effective oil extraction. The material is then heated, to further facilitate oil and fat extraction, then passed through a screw press, which squeezes out the oil.
Additional oil can then be extracted from the remaining material, the oil cake, via solvent extraction using a volatile hydrocarbon, normally hexane. The oil is finally separated from the solvent by distillation and can then be refined: heated and mixed with alkaline substances to remove undesired fatty acids, degummed, bleached and deodorised (figure 3).
Figure 3:
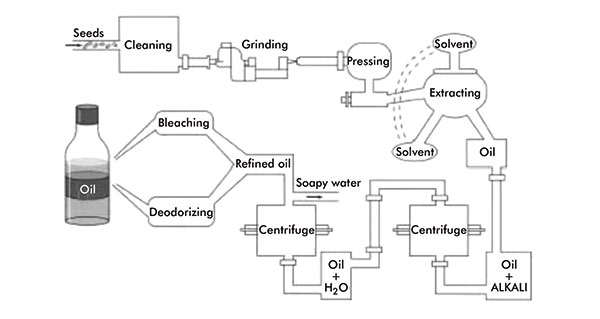
Vegetable oil production
Animal oils, fats & waxes production
Animal fats, oils and waxes are mainly produced by rendering, which is the thermal processing operation that breaks down the cellular structures to release triacylglycerols from animal by-products and underutilised fish species.
There are two methods of rendering: ‘wet’ and ‘dry’ (figure 4). Wet rendering employs the use of a steam injection. The steam heats the animal material to around 95°C, which extracts the oils, fats and waxes.
In dry rendering, the animal material is heated, usually to 115°C–135°C, while being agitated to prevent charring, thus releasing its fats, oils and waxes as it cooks in its own moisture. The fats, oils and waxes sink to the bottom of the vat and can be run off. Wet rendering can be preferable as it produces higher quality extracts, but it is more time consuming.
Other waxes production
Other natural waxes, including beeswax, candelilla wax, carnauba wax, berry wax, sunflower wax, Myrica fruit wax, rice bran wax, lanolin and jojoba oil, have distinct processing methods. Beeswax is extracted by melting the combs using either boiling water, steam or heat; it can then be separated either by skimming off, running off or centrifugal extraction.
Myrica fruit wax is obtained from the fruit peel of the Myrica pubescens tree, by boiling and then skimming the wax off the surface, and lanolin, a “waxy substance secreted by the sebaceous glands of wool-bearing animals”, is derived from wool, which is scoured in hot water with a detergent and the wax removed by centrifugal separators.
Figure 4:
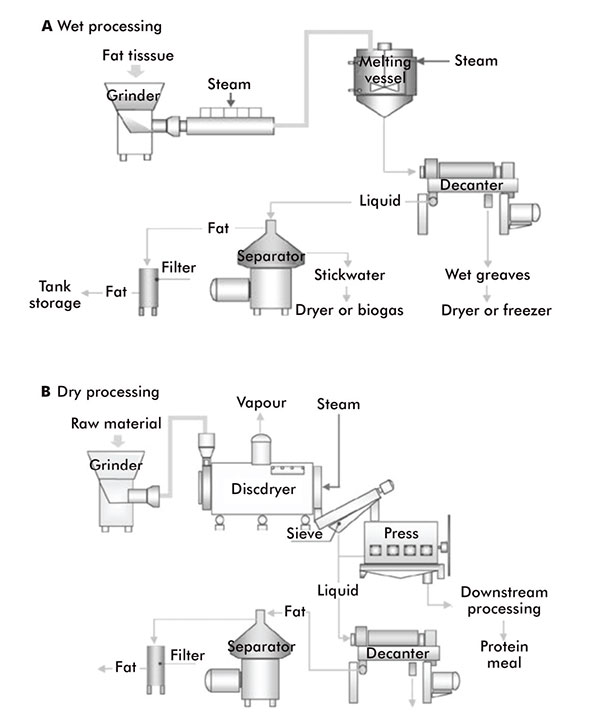
Wet and dry rendering processes
Natural derivatives
In cosmetics, natural derivatives, made from oils and fats, including beef tallow, rapeseed, soybean, coconut palm and oil palm, and the wax lanolin, are incredibly useful raw materials. They can better harness the desired properties of their parent materials, or can provide new functions which were previously unavailable.
In order to create derivatives of oils, fats or waxes, they must first be broken down into their component parts. Once separated, they can be utilised without further processing, or can undergo various modifications, either being recombined in a different configuration, or first modified then recombined.
Natural derivatives: Production
The production of natural derivatives from fats and oils is achieved via various processes, including high pressure splitting, esterification, hydrogenation and ethoxylation.
High pressure splitting is a method by which the fatty acid is separated from the triglyceride via an endothermic reaction, in which oil reacts with a counter-current of water, facilitated by high pressure at around 60 bar and a temperature of around 260°C.
This enables the water to partially dissolve in the fat and hydrolysis to take place. The fat is split from triglyceride to diglyceride, to monoglyceride and then to fatty acid, with glycerine produced as a by-product.
Once separated, the fatty acids can be split into groups of different carbon chain lengths using fractional distillation, and unsaturated and saturated fatty acids can be separated by fractional crystallisation using an organic solvent or hydrophilisation.
The process of wax splitting is very similar; sodium hydroxide solution is used in place of water, with which the fatty acid of the wax ester reacts and forms a soap. When the fatty acid reacts with the sodium hydroxide it splits from the fatty alcohol, which is removed by vacuum distillation.
The remaining soap is then split using a mineral acid and the fatty acid floats to the top of the solution where it is removed.
Hydrogenation is a process that can either be carried out on a fatty acid or a fatty acid ester; it involves the use of an excess of hydrogen and a catalyst, usually nickel, and takes place at a pressure of around 250-300 bar and a temperature of 200-300°C. The hydrogen reacts with the carboxyl group producing a fatty alcohol plus either water, or alcohol respectively.
Ethoxylation is an important process for the production of many natural oil and fat derivatives. It is a condensation reaction in which ethylene oxide reacts with reactive hydrogen molecules in the hydroxyl groups of fatty acids, fatty alcohols and their esters.
Petrochemical-derived oils, fats and waxes can be found in the INCIs of countless cosmetic and toiletry products
Synthetic oils, fats and waxes
Petrochemical derivatives: Origin, structure and production
Petrochemical-derived oils, fats and waxes can be found in the INCIs of countless cosmetic and toiletry products. These raw materials comprise petrolatum, paraffin oil, paraffin wax, microcrystalline wax and ozokerite. All are derived via the same method with the exception of ozokerite, a naturally occurring mineral wax which is mined, extracted by boiling in water, refined using sulfuric acid and then deodorised with charcoal. The other materials are derived through the fractional distillation of petroleum.
An intermediate product in microcrystalline wax production, petrolatum is separated from paraffinic residual oil, via solvent dewaxing and filtration. It is then purified via hydrogenation and/or adsorption to saturate many of the aromatic compounds and remove harmful polar hydrocarbons.
Otherwise known as Vaseline, petrolatum comprises mineral waxes (paraffin wax, microcrystalline wax) and mineral oils (paraffin oils) as a colloidal system, with the waxes forming the solid external phase and the oils the liquid internal phase.
Paraffin oils have a lower molecular weight than petrolatum and mainly comprise a mix of paraffinic and naphthenic structures, at around 70% paraffinic and 30% naphthenic in cosmetic grade oils.
Paraffin wax and microcrystalline wax are components of petrolatum. Paraffin wax, like petrolatum, is colourless, odourless and tasteless. It has a melting point usually between 50°C and 70°C, and depending on the molecular weight can either be hard and brittle (high molecular weight), or malleable (low molecular weight).
Microcrystalline wax generally contains a higher percentage of isoparaffinic and naphthenic hydrocarbons than paraffin wax, and has a higher viscosity and melting point (63-93°C). It has an amorphous structure containing microscopic crystals, which can prove advantageous over the use of paraffin wax, for cosmetic and toiletry products where the crystallisation characteristic of paraffin wax would be a problem.
Ozokerite is chemically and physically identical to microcrystalline wax, and so shares the same properties. All of these petrochemical waxes are primarily formed of straight chain paraffins with carbon chains of 20-32 atoms long.
Silicones: Origin, structure & production
Silicones are synthetic polymers that comprise a silicon-oxygen backbone with organic groups attached to the silicon atoms by C-Si bonds. The most commonly used silicones have methyl groups attached along the backbone. Figure 5 shows the components of these silicones. A variety of silicones are used in cosmetics applications including cyclic, linear, or organo-functional polydimethylsiloxanes (PDMS), as well as silicone elastomer dispersions and resins.
Figure 5:
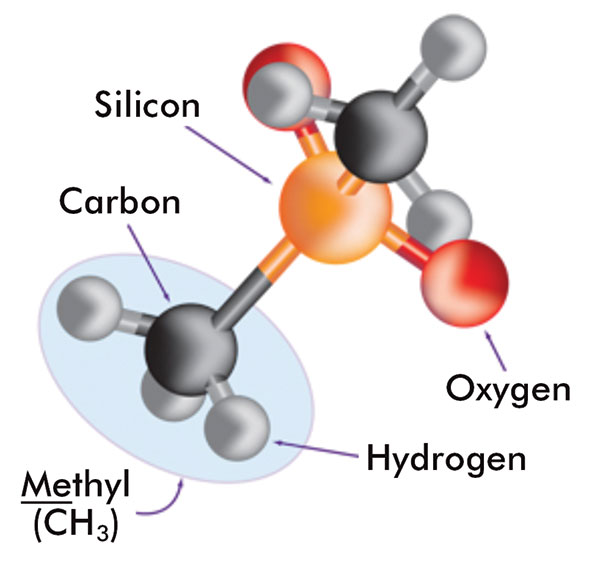
The components of silicone
To produce the silicones used in cosmetics and toiletries, silicon/silicon metal first needs to be extracted from raw materials, such as sand, quartzite and granite rock, because silicon does not occur in its unbound form in nature.
To achieve separation, a carbo-thermic smelting process is employed, in which the reduction of sand at very high temperature releases the silicon. The silicon then undergoes three additional manufacturing processes before becoming silicones.
The first process is chlorosilane synthesis, where silicon is reacted with methylchloride in the presence of a copper-based catalyst to produce chlorosilanes, the most abundant of which is dimethyldichlorosilane. Distillation is then carried out to separate the chlorosilanes. The next process is chlorosilane hydrolysis, which yields silanols.
These cyclic and linear oligomers can then be used without further processing. However, the majority of silicones used in cosmetics and toiletries require a final “finishing process”, ie polymerisation (cyclic) and polycondensation (linear). This enables the creation of the vast array of silicones used in cosmetics and toiletries.
Oils, fats and waxes in cosmetics

Oils, fats and waxes can be used in cosmetic formulations as solubilisers, superfatting agents, consistence factors, consistency factors, emollients and dispersing agents. Natural oils and fats are especially important as they are also used for the production of all classes of surfactants.
The solubiliser polysorbate 20 can be derived from coconut oil and enables fragrance or essential oils to be incorporated into a solution; superfatting agents cetyl and stearyl alcohols are used in hair conditioners; and the consistency factors ozokerite and microcrystalline wax are a frequently used in combination in lipsticks, providing good oil binding and strength.
Emollients lubricate, protect, moisturise and provide a pleasant skin feel. Petrolatum is commonly used as an emollient in a wide variety of products. Cocoa butter and shea butter are examples of commonly used natural emollients, while silicone derivatives dimethicone and cyclomethicone can be used as emollients in products for which a non-greasy feel is desired.
The main problem for natural oils and fats is that their triglyceride components have a propensity to oxidise and hydrolyse
Limitations of oils, fats & waxes
The main problem for natural oils and fats is that their triglyceride components have a propensity to oxidise and hydrolyse. This causes consistency and stability changes, affecting textural qualities and limiting shelf life. Furthermore, oxidation can result in rancidity, causing discolouration and malodours. Sunflower oil has a particular tendency to go rancid due to its high levels of oleic and linoleic acid, which are unsaturated and so prone to oxidation.
Bloom formation is a common problem, caused by the crystallisation of fat at the surface of a cosmetic product. Blooming occurs when the solid fat material dissolves in the liquid phase and migrates to the surface of the product, where it then crystallises, creating an undesirable appearance (figure 6).
Cocoa butter has a tendency to ‘bloom’ due to its temperature sensitivity; at ambient temperature it is solid, but at 36°C it is liquid.
Emulsion stability is also an issue. Even though some fats, oils and waxes have emulsifying properties, eg stearic acid, the majority of emulsions created will at some point split, and so creaming, flocculation, coalescence and phase separation are always a concern.
A limitation specific to natural oils, fats and waxes, arises from their dependence on a source material, which is subject to variability. For example, oil produced from different harvests will have composition variances, and yearly differences in crop yield may cause fluctuations in price and sometimes temporary unavailability of a product.
